POTENTIAL OF NANOTECHNOLOGY IN ENVIRONMENTAL POLLUTION CONTROL
Nanotechnology is a field related to the design, analysis, fabrication, and application of structures, devices, and systems by controlling shape and size at the nanometer scale (nm, 1 nm = 10-9 m). The boundary between nanotechnology and nanoscience is sometimes unclear, but both focus on nanomaterials.
Potential environmental benefits of Nanotechnology
As raw material and energy costs continue to rise, along with increasing environmental awareness among consumers, they also bear responsibility for a range of products on the market that promise certain benefits for environmental and climate protection. Nanomaterials exhibit unique physical and chemical properties that make them appealing for environmentally friendly products.
Examples of these potential benefits include increased durability of materials against mechanical impact or weather, which helps extend the useful life of a product; nano-based anti-corrosion and water-resistant coatings; new insulating materials to enhance the energy performance of buildings; and incorporating nano-particles into materials to reduce weight and save energy during transportation. In the chemical industry, nanomaterials are used based on their special catalytic properties to enhance energy and resource efficiency, and nanomaterials can replace harmful chemicals in certain applications. The potential is being explored in optimizing nanotechnology products and processes for energy production and storage; these are currently under development and are expected to contribute significantly to climate protection and solving our energy issues in the future.
Nanotechnology could make recycling batteries economically attractive: Many batteries still contain heavy metals like mercury, lead, cadmium, and nickel, which can cause environmental pollution and threaten human health if batteries are not disposed of properly. Not only do billions of dollars’ worth of batteries in landfills pose environmental problems, but they also represent a complete waste of a potential and inexpensive raw material. Researchers have found ways to recover pure zinc oxide nanoparticles from alkaline batteries for use in Zn-MnO2 batteries.
Nanomaterials for cleaning radioactive waste in water: Scientists are investigating solutions for radioactive waste treatment using nanotechnology, specifically employing titanate nanofibers as adsorbents to remove radioactive ions from water. Researchers have also confirmed that the unique structural properties of ultraviolet nanotubes and nanofibers provide high-quality materials for removing radioactive cesium and iodine ions from water.
Nanotechnology-based solutions for oil spill incidents: Conventional cleaning techniques are insufficient to address widespread oil spills. In recent years, nanotechnology has emerged as a potential source of new solutions for many of the world's prominent problems. Although the application of nanotechnology to clean up oil spills is still in its early stages, it holds great promise for the future. In recent years, there has been increasing global interest in finding suitable solutions for cleaning oil spills using nanomaterials.
Water treatment applications: The potential impacts of nanotechnology on water treatment applications are divided into three categories: treatment and remediation, detection and monitoring, and pollution prevention. Improving desalination techniques is a major field. Nano-technology water filtration devices have the potential to transform desalination, for example, by using ion concentration polarization phenomena.
A relatively new purification method: brackish water is capacitive deionization (CDI). The advantages of CDI are non-secondary pollution, cost-effectiveness, and energy savings. Nanotechnology researchers have developed a CDI application using graphene-like nanoflakes as electrodes for capacitive deionization. They found that graphene electrodes result in better CDI performance compared to conventional activated carbon materials.
Carbon dioxide capture: Before CO2 can be stored, it must be separated from other gases emitted from combustion or industrial processes. Most current methods for this type of filtration are costly and require chemical use. Nano-techniques for fabricating nanoscale thin films could lead to new membrane technologies that may change this.
Hydrogen production from sunlight – artificial photosynthesis: Companies are developing technologies using hydrogen as part of green, environmentally friendly technologies. While hydrogen fuel is indeed a significant source of clean energy, the challenge is that you cannot just dig a well to get hydrogen; it must be produced, which can be achieved using various resources.
Artificial photosynthesis, using sunlight to split water into hydrogen and oxygen, could provide a clean and sustainable energy source like sunlight. It takes about 2.5 volts to break a single water molecule into oxygen along with negatively charged electrons and positively charged protons. Separating these positively charged electrons and protons from water molecules provides electrical energy.
Research on the nanoscale has shown that a nano-crystalline membrane obtained from inexpensive and environmentally friendly inorganic substances can be combined with a low-cost electrolytic component containing abundant elements to create a low-cost and stable photo-electrochemical hydrogen production system.
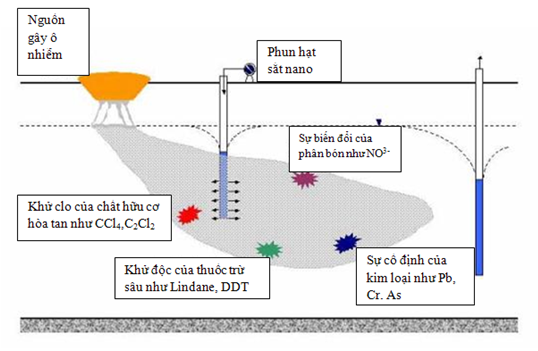
Application of Nano Iron in environmental pollution treatment:
Compared to micro-sized particles, nano-sized iron particles have a higher reaction rate due to their larger specific surface area and active surface area. Furthermore, due to their ability to remain suspended, nano iron can penetrate polluted soils, sediments, and groundwater. However, due to the agglomeration of nano-particles, they are difficult to remain suspended for long periods. Schrick and colleagues demonstrated that a carbon source significantly reduces agglomeration and enhances the transport of nano iron particles.
Nano iron has been found to effectively react with various types of environmental contaminants, including chlorinated organic compounds, heavy metals, and other inorganic substances.
Degradation of chlorinated organic compounds: Nano iron can reduce most chlorinated organic compounds to non-toxic substances such as hydrocarbons, chlorine, and water. Lowry evaluated the dechlorination efficiency of PCBs dissolved in water-methanol solutions using micro and nano-sized iron. With market-available micro iron, no dechlorination was observed after 180 days, whereas nano iron showed the ability to dechlorinate PCBs in a water-methanol mixture under ambient conditions within 45 days.
1. Removal of heavy metal ions
- Arsenic Removal: Kanel and colleagues conducted experiments with varying concentrations of nano iron (0.5; 2.5; 5; 7.5; 10 g/l) to assess the adsorption capacity of As(III) (1 mg/l at pH = 7) on the material's surface. Results showed that, except at a concentration of 0.5 g/l, over 80% of arsenic was adsorbed within 7 minutes, and nearly 99% was adsorbed after 60 minutes. The maximum adsorption capacity based on Freundlich's law was 3.5 mg arsenic/g of nano iron at 25°C.
- Lead and Chromium Removal: Nano iron has been stabilized to separate and retain Cr(VI) and Pb(II) from solutions more quickly, reducing Cr(VI) to Cr(III) and Pb(II) to Pb(0), while oxidizing iron to geolite. Based on experiments with 0.5 g of nano iron and 100 ml or 50 mmol solutions over 8 days, 1 g of nano iron removed 12 mmol of Cr(VI) and 0.18 mmol of Pb(II).
2. Removal of inorganic contaminants
- Selenium Removal: Mondal and colleagues investigated selenium removal using nano iron and synthesized Fe-Ni alloys. In a 5-hour experiment, nearly 100% of selenium was removed by Fe0 nano material. With a material concentration of 0.1 g/l, the selenium removal rate of nano iron was 155 mg/g. At specified concentrations, the selenium treatment efficiency of nano iron increased with the amount of material used.
- Nitrate Removal: Considering the kinetics of nitrate reduction using nano iron, Choe and colleagues demonstrated that complete nitrate reduction in solution could be achieved in just a few minutes by exposing the solution to nano iron powder under ambient conditions without pH control.
(Source: Internet)

 Home
Home